Signaling and Motility
Work Package L5
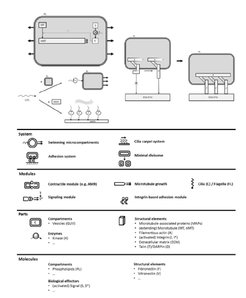
The overall and common goal in Work Package L5 is to synthesize a microcompartment which is able to receive external signals, transmit these signals across the microcompartment wall and translate them into morphological changes and mobility of the microcompartment. To construct a morphogenic membrane system, the Bastiaens Group reconstituted dynamic microtubule asters in GUVs, established a light-controllable protein translocation system in GUVs, and introduced a kinase/phosphatase system to establish a phosphorylation gradient of the microtubule regulator OP18/stathmin to spatially and temporally control microtubule dynamics. Moreover, a conformational stathmin FRET sensor was developed and optimized to monitor spatial phosphorylation gradients. To generate self-organized patterns of phosphoinositides (PIPs), the Bieling Group successfully reconstituted self-organized reaction-diffusion patterns of PIPs on supported lipid bilayers and on the outside of GUVs. To allow spatially and temporally controlled assembly of biological processes, the Spatz Group developed a modular engineering approach based on high-throughput droplet-based microfluidic technology for sequential bottom-up assembly of stable and, therefore, manipulable cell-like compartments. The enhanced stability enabled the sequential loading of such compartments with lipids, transmembrane and cytoskeleton proteins. This approach allowed the development of synthetic cells that are capable to self-assembling different cytoskeletal and transmembrane proteins, and, as a consequence, generating cellular functions such as adhesion, migration and self-propelling. To develop synthetic biology approaches using self-organizing principles to create locally driven fluid transport, the Bodenschatz Group has focused on generation and analysis of synthetic cilia from basic building blocks derived from biomolecules, namely microtubules and molecular motors. In order to achieve the initiation of morphogenic changes and mobility of the developed synthetic cells in response to outside signals, a coherent and synergistic approach is currently being developed between the three partners.
