A stable shell for artificial cells
October 2017
Scientists are developing cell-like lipid vesicles which can be populated with natural cell proteins
Every cell needs a shell. The cell interior is separated from its surroundings by a membrane made up of fat molecules, helping to create the environment needed for the cell to survive. Development of artificial cells is similarly reliant on a chemically and mechanically stable shell. Within the framework of the MaxSynBio network, researchers from the Max Planck Society and the Universities of Heidelberg, Jena, Magdeburg and Bordeaux have used a novel technique to produce particles made of a range of different fatty acids which behave very like natural cell membranes. The scientists were also able to fill the vesicles with natural cell proteins and integrate proteins into the lipid layer. These lipid particles are an important step towards developing a model system for studying processes in natural cells. They could also one day be a component of artificial cells.

© Weiss et al, Nature Materials 2017
At first glance, the natural cell membrane looks like a relatively simple structure consisting of a double layer of fatty acid molecules. But in fact, the cell membrane exhibits properties which have proven very difficult to reproduce in the laboratory. Artificial cells have a shell made of fat molecules; however, until now, it has been too unstable and non-porous. As a result, scientists have been unable to populate these artificial cells with the molecules required for cellular processes to take place.
With the aid of a trick, the Max Planck scientists and their colleagues created lipid vesicles that could in future form the basis for artificial cells. The researchers used droplets made from long-chain organic molecules known as amphiphilic polymers, which act like surfactants. The droplets consist of an outer layer of perfluorinated polyether and an inner layer of water soluble polyethylene glycol to which gold nanoparticles have been attached. The difference in solubility between the inner and outer layer means that the droplets float in an oil-containing medium, while retaining an aqueous solution in their interior. Using a micro-injection system, the researchers were able to inject tiny lipid vesicles into the polymer droplets. Adding magnesium causes the vesicles inside the droplets to dissipate and merge to form a single lipid layer on the inside of the droplet.
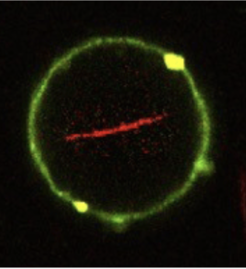
© Weiss et al, Nature Materials 2017
“The lipid vesicles that this produces are mechanically and chemically stable, allowing us to inject proteins into them, as in natural cells,” says Joachim Spatz from the Max Planck Institute for Medical Research in Heidelberg. Using a picoinjection system specially developed for this purpose, the researchers could inject precisely controlled quantities of cellular proteins into the polymer-lipid vesicles. “Using this technique, we are able to populate up to 1000 vesicles per second with proteins – cytoskeletal proteins like actin and tubulin or the transmembrane protein integrin. This means we can quickly obtain enough vesicles for biological or medical analysis,” explains Spatz. The scientists then remove the surfactant shell and transfer the lipid vesicles to an aqueous solution. The vesicles can, for example, then be made to interact with natural cells.
The new technique is not just limited to helping develop artificial cells, as is the goal of synthetic biology and in Germany the MaxSynBio research network of the Max Planck Society. It also offers a simple model system that is quick to manufacture and can be used to study interactions with signalling molecules on other cells or viruses.
The project involved researchers from the Max Planck Institutes for Medical Research in Heidelberg, for Dynamics and Self-Organization in Göttingen, for Dynamics of Complex Technical Systems in Magdeburg and of Colloids and Interfaces in Potsdam, and from the Universities of Heidelberg, Jena, Magdeburg and Bordeaux.
This article was published first in MPG news room.

